
A free sale certificate, also known as a "Certificate for Export" is a document issued by the national regulatory authority. The Central Drugs Standards Control Organization (CDSCO) streamlines the application process for free sale certificate for notified medical devices in India.
The manufacturer can obtain a Free Sale Certificate of regulated /notified medical device through online medical device portal and the certificate shall be issued by CDSCO within 30 working days. Application for the free sale certificate must be filled out online via the Sugam portal with a list of the necessary documents.
Any manufacturer holding a valid manufacturing license can apply for the Free sale certificate from CDSCO.
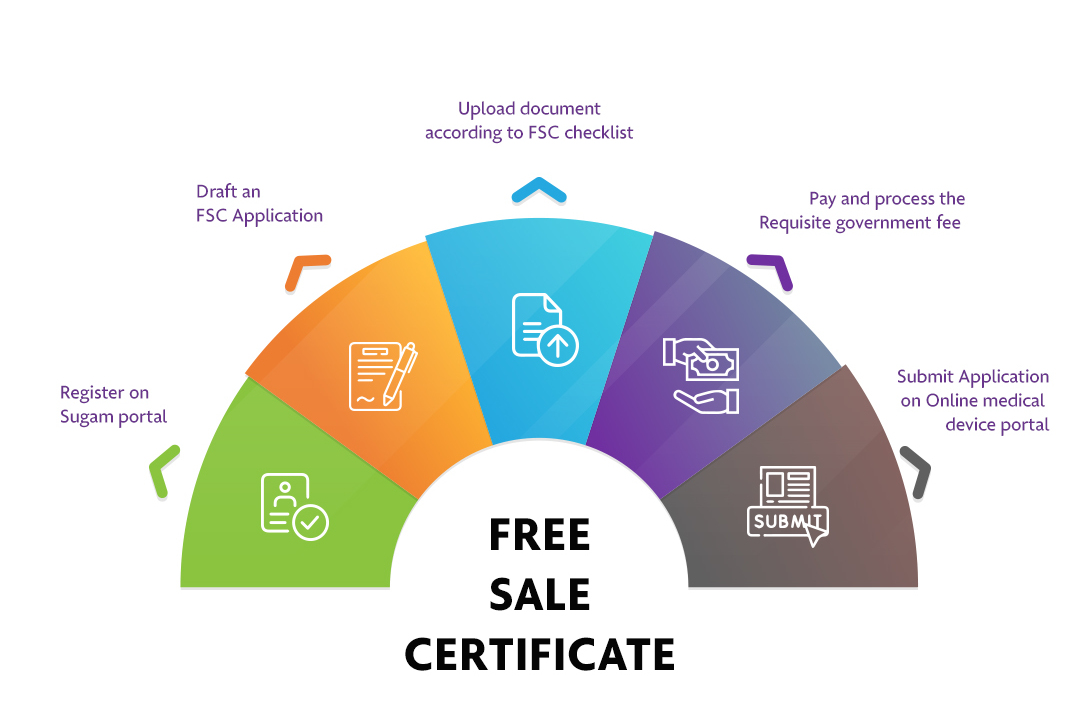
The Applicant must follow the following process:
 |
Step 1: Register on Sugam portal |
 |
Step 2: Draft an FSC Application |
 |
Step 3: Upload document according to FSC checklist |
 |
Step 4: Pay and process the Requisite government fee |
 |
Step 5: Submit Application on Online medical device portal |

A Free Sale Certificate is valid for as long as the Manufacturing license is valid.

FSCfrom Central Drugs Standard Control Organisation
30
WORKING DAYSAll the documents must comply with the FSC checklist.
Manufacturer must have a valid license.
The FSC application process has been made available online by CDSCO.

Each medical equipment requires a fee of INR 1000.

No
