Diagnostic Kits play an important role in Medical science and are the base of almost every test, surgery or medical experiment. Pertaining to their importance and effectiveness in the medical field, the government of India has promulgated proper rules and guidelines for diagnostic kits in the New Medical Devices Rules, 2017, w.e.f 1st January, 2018. All the diagnostic kits whether used In-vitro or In-vivo are now regulated under the New Medical Device Rules, 2017. Diagnostic kits either manufactured in India or imported from foreign countries require to get the license for manufacturing, sale and use in the Indian market from Licensing Authority depending upon their classification.
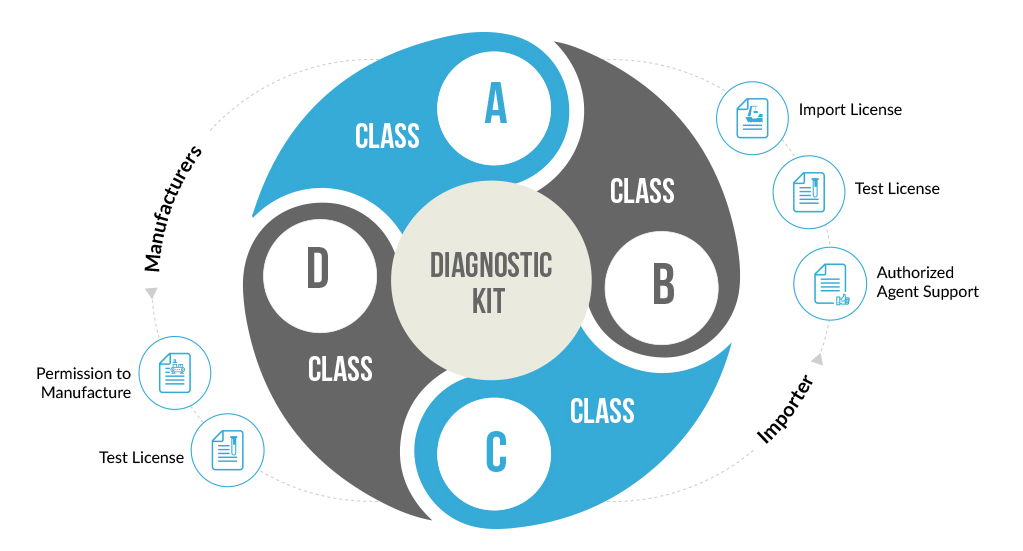
Following the New Medical Device Rules, 2017, all in-vitro diagnostic devices and kits have been classified into four basic categories licenses for which are allotted by respective Central and State authorities. The categorization is based upon the complexity and risk involved with using the diagnostic kit.
Any In-Vitro Diagnostic Device which does not have a predicate or similar device in the Indian market is considered to be a New In-Vitro Diagnostic Device. Such devices need to gain approval from the Central Licensing Authority for their manufacture or Import in India.
Based on New Medical Rules, the classification of the in-vitro diagnostic kits takes into consideration factors like the involved risk, medical condition being diagnosed, self-testing or near patient testing. IVD kits are used for serious medical conditions like HIV or Cancer are classified as high-risk devices and hence placed under Class D. Other simple kits like glucose testing strips, and sphygmomanometers are placed under Class A and B.
In-vitro Diagnostic Kits shall be classified in the following categories.
The classification of the diagnostic kits takes into consideration factors like the involved risk, medical condition being diagnosed, self-testing or near patient testing. Diagnostic kits used for serious medical conditions like HIV or Cancer are classified as high-risk devices and hence placed under Class D. Other simple kits like glucose testing strips, and sphygmomanometers are placed under Class A and B.
CDSCO has provided various forms to be used while filing the application for permission to import or manufacture diagnostic kits. The following table contains specific form which the applicant needs to be filled.
| Applicant | Risk/Class | Type of License | Forms |
|---|---|---|---|
| Importer | A, B, C, D | Importer License | Application: MD-14 Permission: MD-15 |
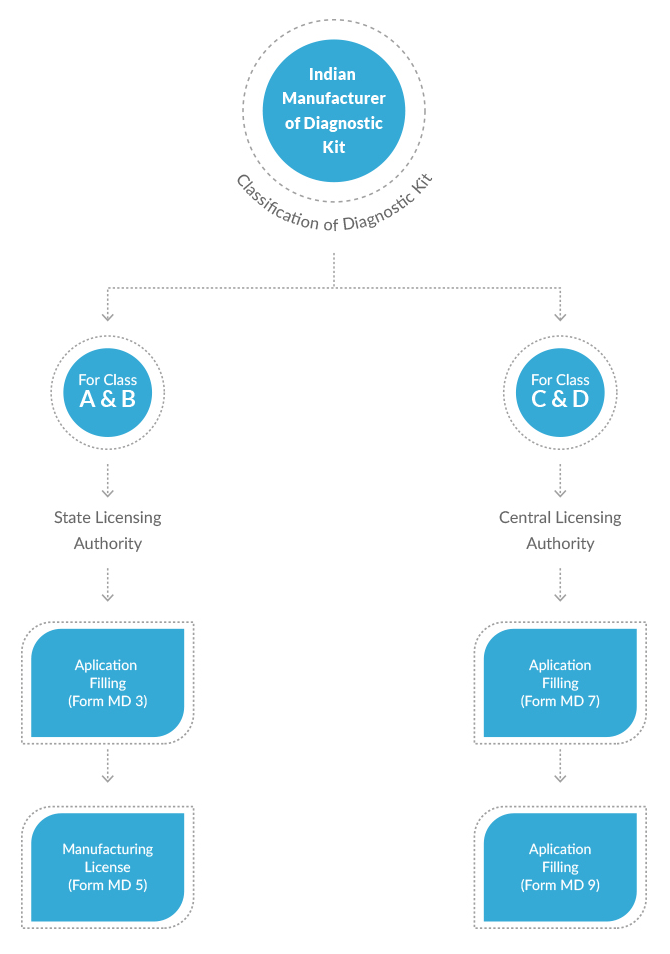
| Manufacturer | A, B | Manufacturing License | Application: MD-3 Permission: MD-5 |
| Loan License | Application: MD-4 Permission: MD-6 |
||
| C, D | Manufacturing License | Application: MD-7 Permission: MD-9 |
|
| Loan License | Application: MD-8 Permission: MD-10 |
||
| Importer | A, B, C, D | Clinical Performance Evaluation | Application: MD-24 Permission: MD-25 |
| Manufacturer | A, B, C, D | Clinical Performance Evaluation | Application: MD-24 Permission: MD-25 |
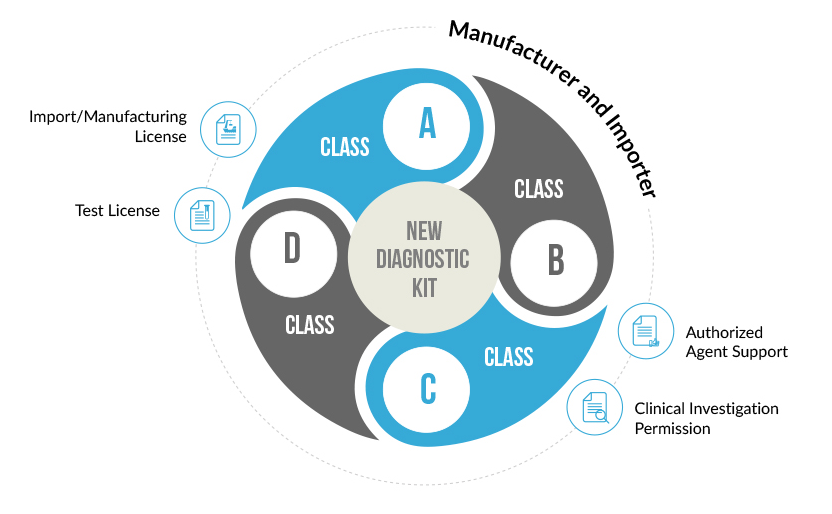
| Importer (New In-Vitro Device) | A, B, C, D | Import License | Application: MD-28 Permission: MD- 29 |
| Manufacturer (New In-Vitro Device) | A, B, C, D | Manufacturing License | Application: MD-28 Permission: MD- 29 |
India has developed at a staggering rate as an economy. Hence the demand for medical care equipment has skyrocketed in the recent years. The new medical rules have classified all the existing diagnostic kits, including those which were earlier not classified. Filing an application for obtaining an import license is a simple single step process now. The process has been simplified to expedite the process and ensure proper availability of diagnostic kits in the country. The New Medical Device Rules allow multiple importers of a single diagnostic kit which was forbidden earlier. Although, each importer has to file a separate application for each diagnostic kit being imported.

Classification
of Medical Devices


Aplication Filing
(Form MD- 14)

Import Licence
(Form MD- 15)
New Medical Device Rules, 2017 have defined distinct provisions to obtain permission for manufacturing of diagnostic kits in India. Different diagnostic kits have been classified under different categories based upon their use, complexity and the risk involved. Applications for Class A and Class B diagnostic kits are reviewed and granted permission by the State Licensing Authorities. Whereas applications for Class C and Class D medical devices are reviewed and granted license by the Central Licensing Authority. This difference is obviously due to the depth and scale of review involved for different classes of diagnostic kits. CDSCO has also defined different fees for different classes of diagnostic kits.
New In- Vitro Diagnostic Device are the devices that has not been approved for manufacturing or importing by Central Licensing authority
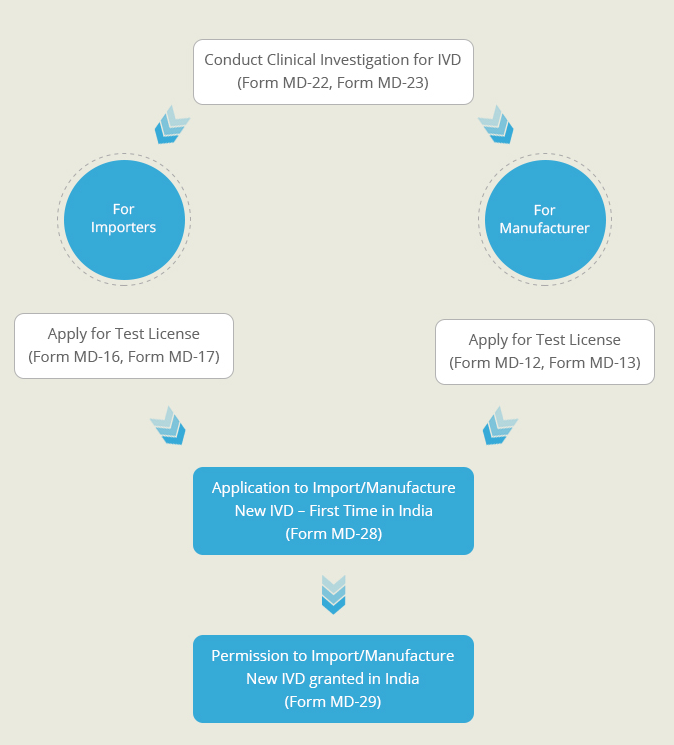
A New In-Vitro diagnostic kit is one whose similar or predicate device is not available in India. Such diagnostic kits need to undergo clinical investigations to prove their safety and effectiveness. This Clinical Performance Evaluation is conducted on specimens collected on voluntary human participants. Once the clinical performance evaluation has been completed a report describing the results of the investigation is generate. An application for the import or manufacture new In-Vitro Device is filed at the Central Licensing Authority, along with this performance evaluation report. After proper evaluation of the report’s findings, a permission is granted by the CLA to import or manufacture the diagnostic kit in India.

Insight
National e-Governance plan (NeGP), launched in 2006 as initiative that Government of India has implemented to make all government services accessible online.
Read More
Insight
Indian Government has chosen to join the foray and ride the digital wave through SUGAM, launched on 14 November, 2015
Read MoreNowadays, therapeutic treatment based on medical devices is providing technologically advanced solutions for the management, diagnosis, treatment,mitigation or prevention of several diseases. Thus, the demand continues to grow in the market at a tremendous rate leading to a renewed interest in the scientific development and research in the field of […]
Read MoreThe Indian government has brought about some major changes with regards to the rules and regulations governing the manufacturing to enhance the quality of products used in the healthcare industry. As India being a major market for the healthcare-related products and its services, these modifications to the existing regulations are […]
Read MoreImport and Registration of Medical Devices, Registration certificate of Critical / Notified Diagnostic Kits, Test licence for Medical Device and Diagnostic Kits, Import License for Diagnostic Kits, Manufacturing licence for medical device, Clinical Trial Approval for Medical Devices and Diagnostic Kits
Read More