
A manufacturer who wants to manufacture in vitro diagnostics medical devices is eligible to apply.
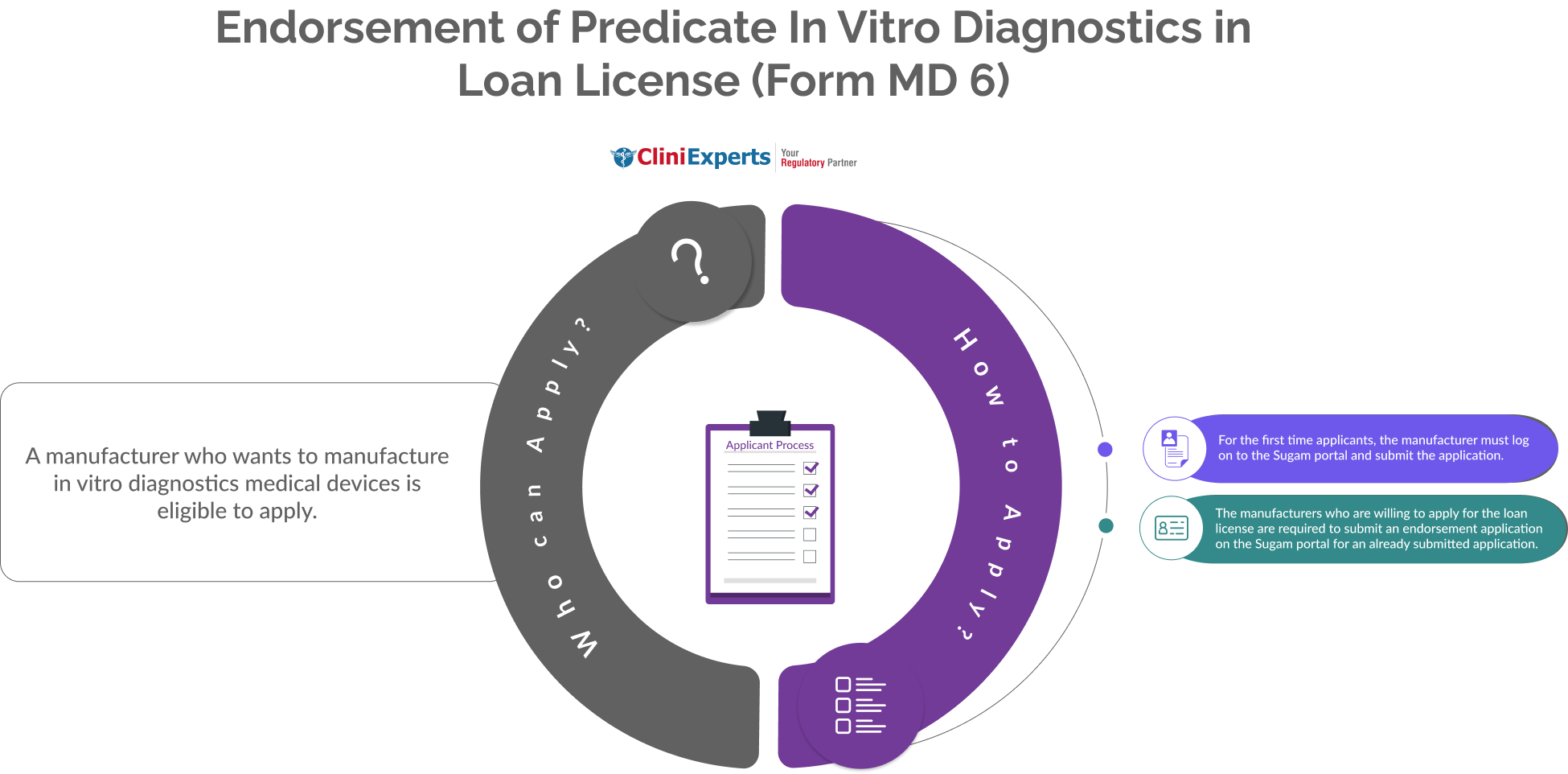
The Applicant must follow the following process:
 |
For the first time applicants, the manufacturer must log on to the Sugam portal and submit the application. |
 |
The manufacturers who are willing to apply for the loan license are required to submit an endorsement application. This is done on the Sugam portal for an already submitted application. |

Unless it is suspended or revoked by the SLA, the manufacturing license in Form MD-06 will remain valid indefinitely. It is subject to the payment of the license retention fee as stipulated in the second schedule. This must be done before the completion of five years from the date of issuance.

MD-06from Central Drugs Standard Control Organisation
4-5
MonthsAccording to our experts, clients should look out for the following the license application procedure:
There must be alignment between the product name, model number, label, IFU, and other technical documentation.
For grouping products, it is vital to adhere to the grouping guidelines on medical devices issued by the MoHFW, Government of India. If you fail to do the needful, you may be charged additional fees by the Government.
Specimen collection tubes are regulated under the definition of specimen receptacle‖ as specified in Sub-clause (zs) of Rule 3 of MDR-2017 and are classified as Class A as per First Schedule, Part II (2(v)(3)) of MDR-2017.

Yes. In – Vitro Diagnostic devices for HBsAg, HIV and HCV manufactured / Imported under valid license issued by the CLA or SLA, may also be used in Blood Bank, as the criteria like Sensitivity (%) and Specificity (%) for evaluation of the HBsAg, HIV and HCV diagnostic kits for the Transfusion purpose (Blood Banks) and Diagnostic purpose are same, Provided the manufacturer claims in the product labels or in the IFU that the product is intended both the purposes; for blood screening and diagnostic.

For multiple brand names of a product, the firm needs to submit applicable product fee as per Second schedule for each of the Brand name.


Yes, IVD products which are currently registered in India have to be registered according to the provisions of Medical Devices Rules, 2017.
