
The application is open to any manufacturer of Medical Devices in India.
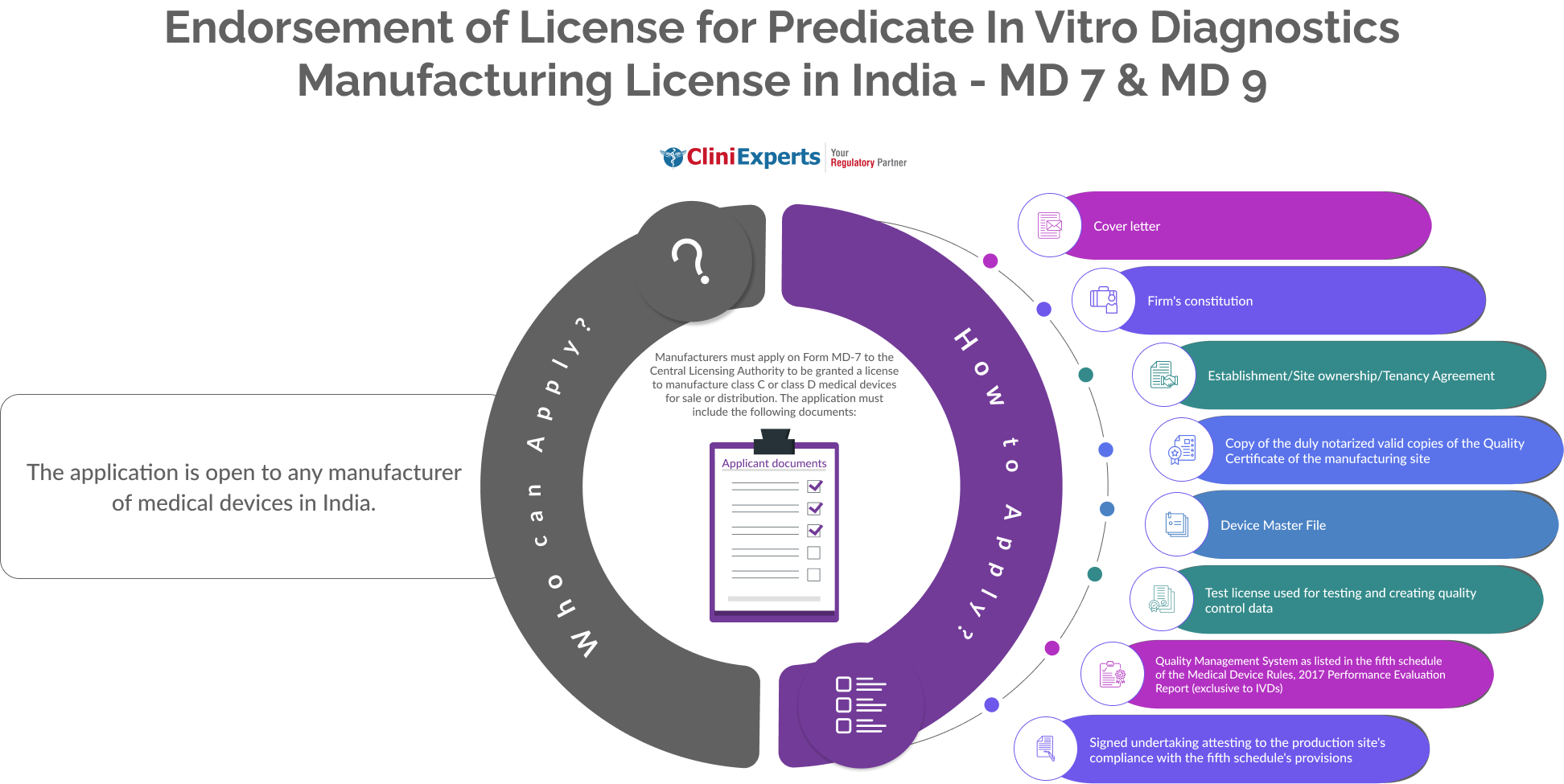
The Applicant must follow the following process:
 |
Prepare Application Form: Begin by filling out Form MD-7, which is required to apply for a Grant of License to Manufacture for Sale and Distribution of Class C or Class D medical devices. |
 |
Assemble Required Documents: Cover Letter, Include Constitution details of the firm ,provide proof of Establishment/Site ownership or a Tenancy Agreement, attach a notarized copy of the Quality Certificate concerning the manufacturing site, Plant Master File, Device Master File etc. |
 |
Include Additional Documentation: Prepare and attach the Plant Master File. Include the Device Master File detailing the medical device specifics. Attach documentation of the Quality Management System as specified in the Fifth Schedule of the Medical Device Rules. |
 |
Special Documentation for IVDs: If applicable (for In Vitro Diagnostic devices only), include a Performance Evaluation Report. |
 |
Compliance Undertaking: Include an Undertaking signed by an authorized representative, stating that the manufacturing site complies with the provisions of the Fifth Schedule. |
 |
Submission of Application: Submit the completed Form MD-7 along with all the attachments to the Central Licensing Authority. |
 |
Inspection and Review: Following your submission, the Central Licensing Authority will conduct a review of the application. This includes an inspection of the manufacturing site for Class C and D medical devices, which may be carried out by Medical Device Officers, with or without an expert or a Notified Body. |
 |
Receipt of License: Upon successful review and inspection, an Endorsement Manufacturing License in Form MD-9 will be issued by the Central Licensing Authority. |


MD 9from Central Drugs Standard Control Organisation
4-6
MonthsAccording to the experts, the manufacturers should produce the quality control data using a valid test license at the time of application submission.
The applicants can expect the inspection by CDSCO within sixty days from the date of submission of the application.


Class C and D licenses will be inspected by the Central Licensing Authority within 60 days of the application date. If the Central Licensing Authority is satisfied that the requirements of these rules have been met, it may grant a license 45 days after receiving the inspection report.

If Class B, C, and D Medical Devices are to be manufactured, a signed undertaking attesting to the production site's compliance with the fifth schedule of Medical Device rules, 2017 must be presented.

