CliniExperts - Your reliable partner for Comprehensive Compliance Solutions
REACH US
CliniExperts is the catalyst you need to speed up your registration process without worrying about obstacles. Our experience, professional relations understanding and wealth of practical familiarity in the field serve to ease your way through the process. With CliniExperts, you will receive expert advice, 24 hour support, practical guidance and complete technical assistance right from the beginning till the very end of your penetration into the Indian market.
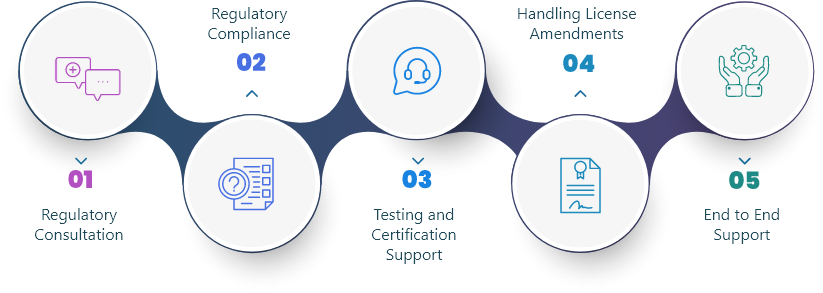

We offer regulatory solutions to Medical Device, Drug, Biologicals, Food, In-vitro Diagnostic and Cosmetics

Regulatory bodies are organizations that have the responsibility to scrutinize, steer and manage different sectors in order to ensure the safety of consumers. Such bodies are formulated by the Government in sectors like the drug industry, cosmetic industry and food industry to regulate and control the quality of products that are made accessible to consumers through the market. Brief descriptions of some apex regulatory bodies that govern the Indian markets are:

The Central Drugs Standard Control Organization (CDSCO) is a regulatory body that lays down stringent standards that any drug, medical devices, diagnostic kits, cosmetics, biologicals and vaccines being introduced into the country must meet before being sold in the market. CDSCO has 6 Zonal Offices which are headed by Dy. Drugs Controller (India).
The State Food and Drug Administration has branches in every state that serve to maintain safety and quality standards of food ingredients, food products and imported drugs. This is the highest governing authority for all food and drug clearances needing entry into the country and every state.
One of the key responsibilities of the CDSCO is to grant permits for specific crucial categories of drugs, including blood and blood products, intravenous fluids, vaccines, and sera. It ensures that these specialized drugs meet the necessary standards and are safe for use.
Its regulatory oversight helps to build trust and confidence in the healthcare system.
The Food Safety and Standards Authority of India (FSSAI) is a branch of the Ministry of Health and Family Welfare dedicated to standardizing food products made available in the market through a series of standards that ensure food safety.
It was created under the Food Safety and Standards Act, 2006 to ensure the safety and quality of food products in India.
The primary responsibility of FSSAI is to regulate and supervise the manufacturing, processing, distribution, sale, and import of food items to ensure they meet the defined standards for safety and quality. FSSAI sets food safety standards, and regulates the licensing and registration of food businesses in India.
The Indian Council of Medical Research (ICMR) is the apex body in India for the formulation, coordination, and promotion of biomedical research. ICMR operates under the Department of Health Research, Ministry of Health and Family Welfare, Government of India.
It is the leading body regulating all medical research in the country. It essays a vital role in the prevention and control of communicable diseases, research regarding toxicology, immunology, reproduction, national medical statistics, food standards and other primary health topics of national interest.
The primary mandate of ICMR is to promote and coordinate medical research activities across the country. It plays a pivotal role in advancing scientific knowledge and addressing health challenges by conducting research, establishing research institutions, and promoting collaboration among researchers, institutions, and industries.
The Department of Scientific and Industrial Research (DSIR) is an apex body in India that regulates and standardizes topics concerning scientific and industrial sectors of national importance. It also holds the power to grant recognition to public funded universities and is the controlling power over all the IITs and IISCs.
DSIR’s initiatives aim to create an environment conducive to research and innovation, strengthen India’s scientific capabilities, and foster the growth of industrial sectors through technological advancements. By promoting R&D and facilitating technology transfer, DSIR contributes to the overall development of science, technology, and industry in the country.
The Directorate General of Foreign Trade (DGFT) is an agency of the Government of India that operates under the Ministry of Commerce and Industry.
The primary role of DGFT is to promote and regulate foreign trade in the country. It is responsible for issuing and regulating export-import licenses. It grants various licenses and authorizations, such as Importer-Exporter Code (IEC), Advance Authorizations, Export Promotion Capital Goods (EPCG) scheme, and Duty-Free Import Authorization (DFIA), among others.
It plays a pivotal role in granting licenses and authorizing trade transactions between India and other countries. It also administers various export promotion schemes to support and incentivize Indian exporters. It conducts investigations, audits, and inspections to prevent trade malpractices, monitor export-import transactions, and enforce compliance with trade laws.
The National Pharmaceutical Pricing Authority (NPPA) is an organization in India that operates under the Ministry of Chemicals and Fertilizers.
The primary objective of NPPA is to regulate and control the prices of pharmaceutical drugs in the country. It has been set up by the government to control and regulate standardized prices for the drugs available in the Indian market.
It ensures that drugs that are needed by masses, and all essential drugs are affordable and easily available throughout the country. It works towards ensuring a balance between the interests of the pharmaceutical industry and the affordability of medicines for patients.
Central Insecticide Board and Registration Committee(CIBRC) is a division of Directorate of Plant Protection, Quarantine and Storage. CIBRC was set up by the Department of Agriculture and Cooperation in order to standardize the quality of insecticides available in the country in order to prevent danger. It ensures that all tenements of the Insecticide Acts and Rules are followed meticulously.
In the Act and the Rules framed there under, there is compulsory registration of the pesticides at the Central level and license for their manufacture, formulation and sale are dealt with at the State level. With the enforcement of the Insecticides Act in the country pesticides of very high quality are made available to the farmers and general public.
The Central Bureau of Narcotics (CBN) is an Indian government agency that operates under the Ministry of Home Affairs. It is responsible for the enforcement of the Narcotic Drugs and Psychotropic Substances (NDPS) Act in India.
The CBN issues licenses for the cultivation, production, and manufacturing of narcotic drugs and psychotropic substances for medical and scientific purposes. It conducts investigations, arrests, and seizures related to drug trafficking, production, and distribution.
It plays a vital role in curbing the illicit drug trade, preventing drug abuse, and protecting public health and safety in India.
your go-to Regulatory Solutions provider in India!
Established in 2009 by Dr. Ashwini Kumar, CliniExperts is a leading provider of Regulatory and Clinical Research Services. With ISO 9001 and ISO 27001 certifications, we prioritize strict adherence to all the rules and regulations and ensure top-notch standards in our services.
Our team of passionate and skilled professionals collaborates with global companies known for their high-quality products, services, and customer care. We specialize in assisting companies across various sectors, including Medical Devices, In-Vitro Diagnostics, Pesticides, Insecticides, Drugs and more.
At CliniExperts, we live by our belief in unwavering quality and pursuit of excellence. Our zero-tolerance policy for defects creates a flawless regulatory ecosystem, enhancing ease of doing business for our clients.
Choose CliniExperts as your trusted partner for regulatory compliance and streamlined approvals.
End-to-End Regulatory Solutions for Domestic and International Markets
Certifications ISO 9001:2015 and 27001:2013




We collaborate with companies operating in various sectors such as pharmaceuticals, biologics, medical devices, in-vitro diagnostics, food, nutraceuticals, cosmetics, and other related industries.
Please feel free to talk to us if you have any questions. We endevor to answer within 24 hours.


