The pharmaceutical industry relies heavily on advanced technology to meet increasing demands. According to a recent report by IQVIA, global pharmaceutical spending is expected to exceed $1.5 trillion by 2023. This growth emphasizes the need for efficient and reliable pharmaceutical equipment that can streamline production processes while ensuring quality.
Key equipment like tablet press machines and sterilizers play vital roles in maintaining product integrity. Studies show that the right machinery can improve production rates by up to 30%. However, the industry faces challenges such as equipment obsolescence and high maintenance costs. These factors can hinder productivity and necessitate a reevaluation of current systems.
Investing in modern pharmaceutical equipment is a path towards improved efficiency. While advancements are significant, existing gaps in user training and operational protocols often lead to inefficiencies. Addressing these shortcomings is essential for maximizing the benefits that modern equipment can provide.

In the pharmaceutical industry, equipment plays a vital role in ensuring efficient production. High-quality mixing equipment, for example, is essential for combining active ingredients evenly. A poorly calibrated mixer can lead to inconsistent dosages, affecting product effectiveness. Proper maintenance and checks are crucial for this equipment to function optimally.
Another important piece of equipment is the filtration system. Efficient filtration ensures the removal of impurities, helping to maintain product integrity. Delays or inefficiencies in filtration can prolong production timelines. For some setups, this can become a costly issue. The choice of filter media can directly impact the efficiency of this process.
Packaging machinery is also key. It must efficiently seal and label products, preserving their quality. Packaging errors might result in increased waste. It’s vital to regularly assess the machinery for any signs of malfunction. Investing in reliable equipment is crucial, but constant evaluation and adaptations to processes are equally important.
Pharmaceutical manufacturing is evolving rapidly. Key technologies are emerging to boost efficiency. Automation is one major technology transforming production lines. Automated systems reduce human error. They streamline processes, saving time and resources. Robotics can handle tasks like packaging and labeling with precision.
Data analytics is another vital technology. It helps manufacturers monitor production in real-time. These insights can lead to better decision-making. However, companies sometimes struggle to interpret data effectively. Misinterpretation can lead to setbacks and unexpected costs. Continuous training in data analysis is essential.
Continuous manufacturing also plays a crucial role. It allows for ongoing production without stops. This method reduces waste and enhances productivity. Yet, implementing continuous systems can be challenging. Legacy systems often hinder integration. Change is necessary, but the transition can be daunting. Embracing these technologies can drive efficiency, but constant evaluation is needed to truly benefit.
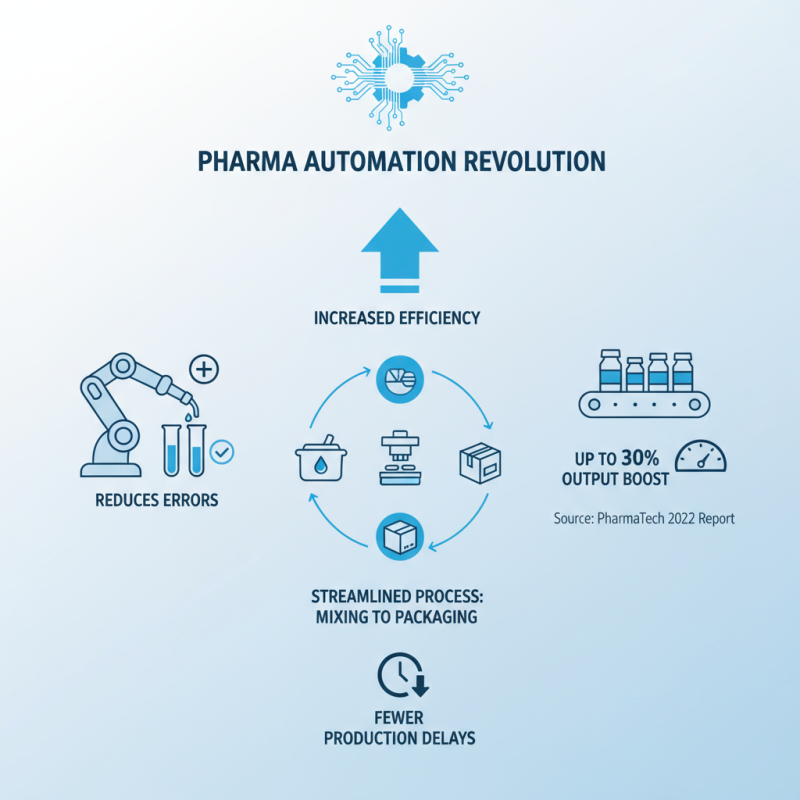
Automated systems are transforming the pharmaceutical production landscape. These systems increase efficiency and reduce errors. A 2022 report from PharmaTech indicates that automation can boost output by up to 30%. This statistic underscores the significance of embracing technology in manufacturing processes. Companies adopting these systems have reported fewer production delays. A well-integrated automated system can streamline everything from mixing to packaging.
However, the integration of automated technology isn't without challenges. Staff training is essential. A Pew Research survey found that 40% of workers feel unprepared for automation. This gap can create resistance to implementing new systems. Additionally, over-reliance on automation can lead to oversight. If processes fail to be monitored closely, it may result in significant product quality issues. Balancing automation with human oversight is crucial for sustainable production.
Moreover, initial investment costs can be a barrier. While long-term gains are evident, the upfront financial commitment can be daunting for many. According to an industry analysis, companies see an average ROI of 150% within three years of automating. Still, the reluctance to invest can hinder potential growth. Thus, understanding both the advantages and challenges of automation is vital for future breakthroughs in pharmaceutical production efficiency.
Quality control is crucial in pharmaceuticals. Innovative equipment plays a key role in ensuring product safety and efficacy. New technologies are helping manufacturers streamline processes and maintain high standards.
Automation is a game changer. It reduces human error and increases consistency. More precise measurements lead to better outcomes.
Tips: Regularly calibrate equipment. This helps maintain accuracy over time. Training staff on new systems is equally important. They need to understand the tools to utilize them effectively.
Recent advancements include real-time monitoring. Sensors can detect deviations in processes instantly. This allows for quick corrective actions. However, reliance on technology can also present challenges. Unexpected failures can disrupt production schedules. Reacting swiftly is essential to minimize downtime.
Tips: Always have contingency plans. This helps the team respond to equipment issues faster. Foster a culture of continuous improvement. Encourage feedback from the team on equipment usability. They are often the first to notice flaws in processes.
In the pharmaceutical industry, regulatory standards shape equipment selection significantly. Compliance with Good Manufacturing Practices (GMP) is crucial. According to a 2022 report by the International Society for Pharmaceutical Engineering, about 90% of manufacturers faced challenges meeting these standards. A failure to comply can lead to costly fines and product recalls.
Equipment must adhere to strict specifications. Regulatory bodies mandate validation processes that ensure reliability and safety. For instance, the U.S. Food and Drug Administration emphasizes rigorous testing for equipment used in drug production. This includes cleaning validation and change control measures. Many companies struggle with maintaining traceability in their equipment, which can lead to lapses in compliance.
In recent years, the push towards automation has gained traction. Automated systems can reduce human error and improve efficiency. However, integration with existing systems presents challenges. A report by Deloitte noted that 70% of companies reported difficulties in transitioning to automated technology. Ensuring that all equipment meets evolving standards is an ongoing battle for many manufacturers.
| Equipment | Function | Regulatory Standards | Key Features |
|---|---|---|---|
| High-Performance Liquid Chromatography (HPLC) | Separation and analysis of compounds | FDA, EMA | Precision, sensitivity, automation |
| Automated Aseptic Filling Systems | Filling of sterile pharmaceuticals | cGMP, ISO 13485 | Minimal contamination, high speed |
| Tablet Press Machines | Manufacturing of tablets | FDA, EP | Efficiency, adjustable pressure |
| Lyophilization Equipment | Freeze drying of pharmaceuticals | FDA, cGMP | Control of temperature and pressure |
| Granulation Equipment | Granule formation | FDA, ICH | Versatility, scalability |
| Blister Packaging Machines | Packaging of tablets/capsules | FDA, cGMP | Tamper-evident sealing, efficiency |
| Mixers and Blenders | Mixing of raw materials | cGMP, ISO 9001 | Uniformity, ease of cleaning |
| Cleaning-In-Place (CIP) Systems | Internal cleaning of equipment | FDA, cGMP | Automated cleaning process |
| Environmental Monitoring Systems | Monitoring of cleanroom environments | FDA, ISO 14644 | Real-time data tracking |
| Quality Control Labs | Testing of pharmaceutical products | FDA, cGMP | Comprehensive testing capabilities |